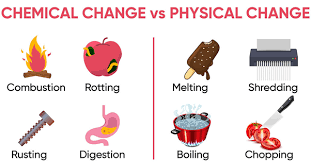
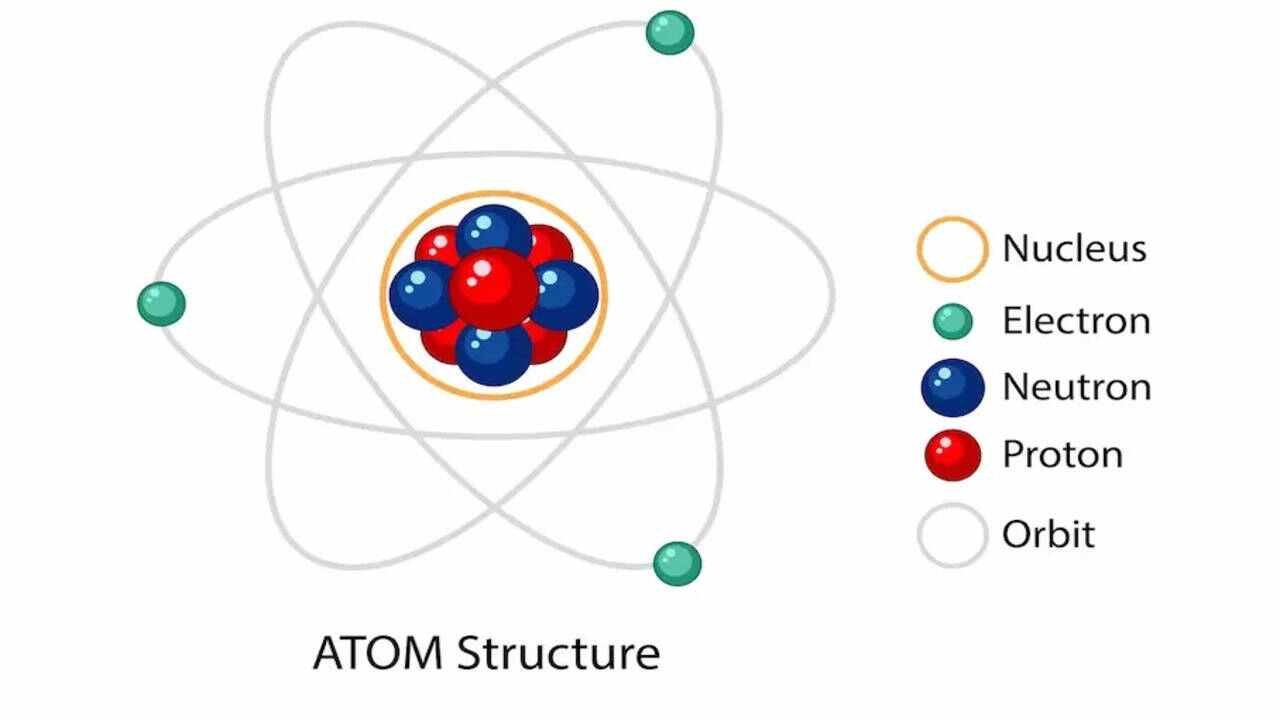
Comprehension
Atoms contain up of 3 basic components known as subatomic particles, consisting of protons (positively charged), neutrons (no charge), and electrons (negatively charged). These are the parts of the atom. The atomic structure of these building blocks is very interesting. The protons and neutrons are located in the center of the atom, while the electrons are quite far from the center. The number of protons in the nucleus of an atom determines its atomic number and its identity as a specific element. For example, all hydrogen atoms have one proton in their nucleus, while all carbon atoms have six protons. The number of neutrons in the nucleus can vary, but the number of protons and neutrons usually add up to the atomic mass of the atom. Atoms are constantly interacting with each other and can combine to form molecules, which are the building blocks of most of the matter that we encounter in our everyday lives.